Where Does Battery Acid Come From

This liquor is called an electrolyte.
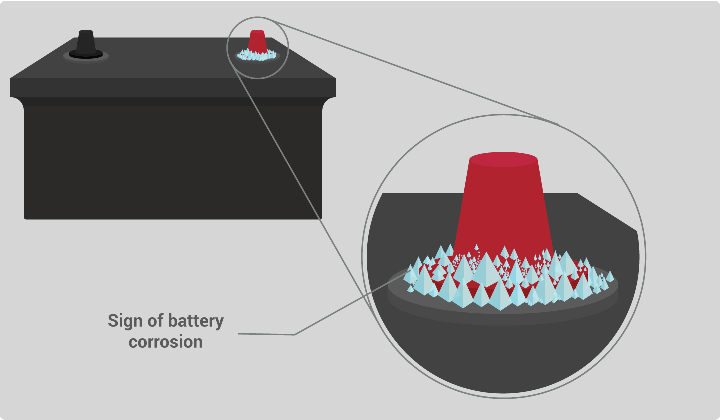
Where does battery acid come from. If this happens surface deterioration will probably be visible. The unused acid will keep just fine in the jug it came in. Excessive sulfation can increase the possibility of acid boiling over spilling the sulfuric acid solution out of the battery.
Symptoms of Battery Acid on Skin. Battery leakage commonly known as battery acid is nasty corrosive stuff it can burn your skin contaminate soil and of course ruin whatever device it has leaked into. But neither is right because this liquid includes both water 50-70 and acid 30-50.
NiMH batteries or nickel-metal-hydride batteries are one of the best available rechargeable batteries in the market. To reduce the effects of the acid immediately neutralize the acid by flushing the area with a solution of baking soda and water. Topping off with new acid in a used battery is a great way to ruin a battery.
Sulfation is when lead sulfate crystals form on the surface of battery plates. If however the sulfuric acid was exposed to human flesh undiluted it would result in immediate burning of human tissues and other organic materials. Sometimes as batteries age they start to leak.
For household batteries this acid is actually alkaline thanks to the potassium hydroxide chemical make-up. Battery acid could refer to any acid used in a chemical cell or battery but usually this term describes the acid used in a lead-acid battery such as those found in motor vehicles. The most fizzing will come from the concentrated sulfuric acid then dilute sulfuric acid then the acetic acidThe amount of fizzing is due to the concentration of H in the solution and.
For lead batteries sulfuric acid is the dangerous residue which requires a different type of clean-up. Battery acid is a solution of sulfuric acid and water diluted which means the dilution reduces the strength of the acid. To clean a gadget caked with the aftermath of a leaking battery dip a cotton swab in an acid such as lemon juice or distilled white vinegar and dab it on the potassium carbonatethat.